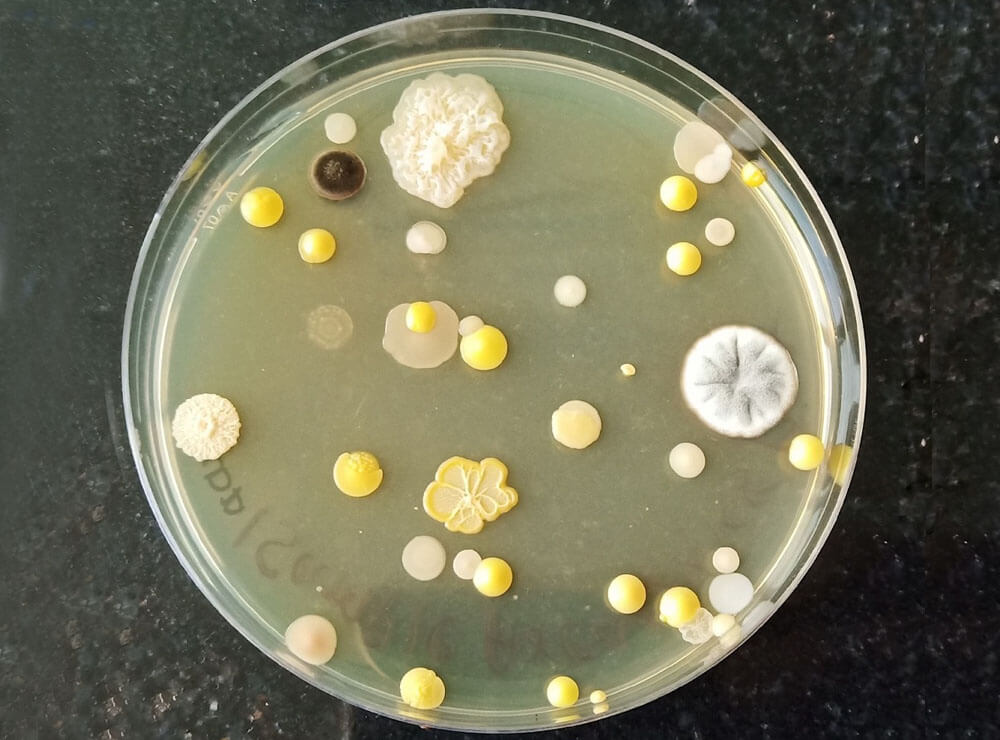
Microbiological Monitoring
Air-borne biological agents such as bacteria and mould can cause serious health problems as well as contaminate product and process and damage to high revenues.
Similarly, personnel are major source of microbial contaminants manufacturing and testing areas therefore personal hygiene monitoring is required on regular basis to ensure product and process are under contamination control state.
Environment, Water, Air, Gases, Personnel
Environmental Monitoring 90mm Plate Personal Monitoring 55mm Contact / RODAC Plate Surface Monitoring Swabs / 55mm Contact Plate Personal Hygiene Monitoring Swabs / Plate
- Area Qualification
- Fogging Validations
- Microbiological Qualifications
- Gamma Irradiation Qualification
Method Development / Suitability / Feasibility Study
Test method development play an important role that method is workable or not to that particular product system.
- Solution Stability / Ad-mixture / Adventitious contamination study
- Burkholderia cepacia complex test (BCC)
- Antibiotic Assays - Test Method Development & Validated Excel Sheet Development
- Sampling, Transport & Storage Study
- Shelf life / Stability Assessments
- Diagnostic Kit Qualification & Equivalency Study
- Medical Device Reprocessing Study

Location
Indore, Madhya Pradesh, India.

Call Us
+91-9949411776

E-mail Us
contact@microbiqlab.com
